

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (Sus scrofa) | BDBM50105033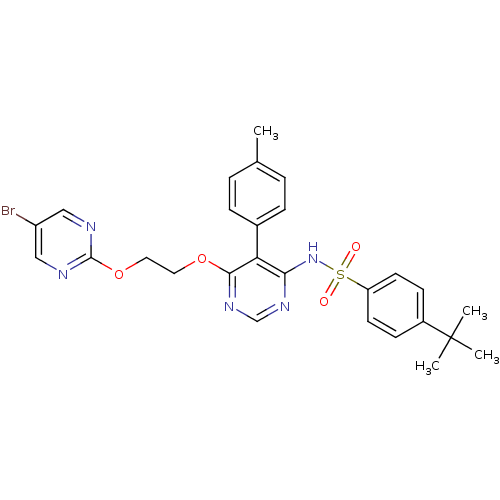 (CHEMBL112531 | N-{6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co. Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [125 I]ET-1 binding to Endothelin A receptor in porcine aortic membrane. | Bioorg Med Chem Lett 12: 81-4 (2001) BindingDB Entry DOI: 10.7270/Q2VX0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50105035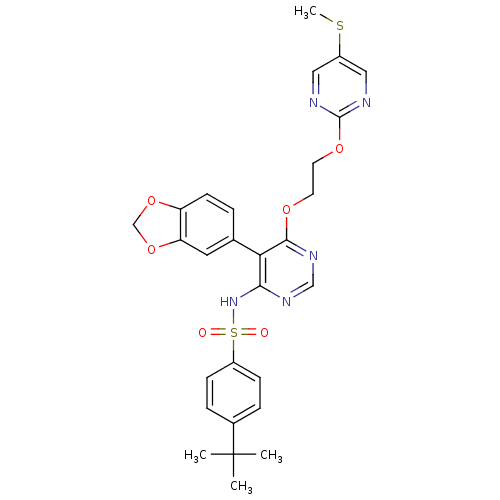 (CHEMBL325511 | N-{5-Benzo[1,3]dioxol-5-yl-6-[2-(5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00110 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [125I]-ET-1 binding to Endothelin A receptor in porcine aortic membrane from endothelial cells | J Med Chem 44: 3355-68 (2001) BindingDB Entry DOI: 10.7270/Q2QN662B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50105034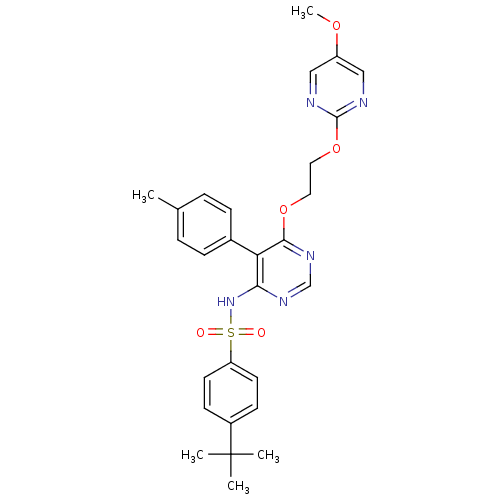 (4-tert-Butyl-N-{6-[2-(5-methoxy-pyrimidin-2-yloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00170 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [125I]-ET-1 binding to Endothelin A receptor in porcine aortic membrane from endothelial cells | J Med Chem 44: 3355-68 (2001) BindingDB Entry DOI: 10.7270/Q2QN662B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50104997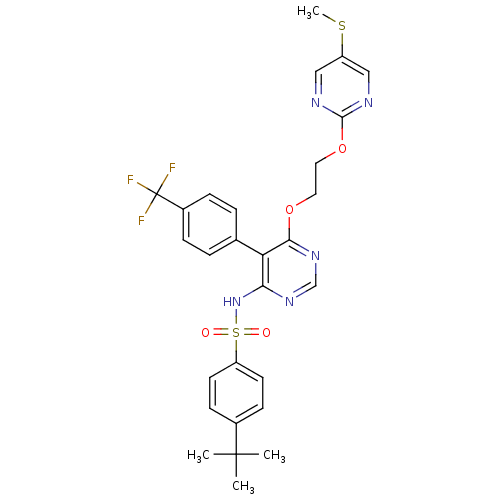 (4-tert-Butyl-N-[6-[2-(5-methylsulfanyl-pyrimidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00170 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [125I]-ET-1 binding to Endothelin A receptor in porcine aortic membrane from endothelial cells | J Med Chem 44: 3355-68 (2001) BindingDB Entry DOI: 10.7270/Q2QN662B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50107562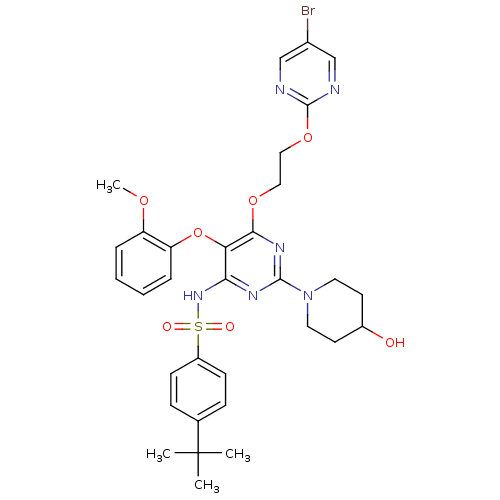 (CHEMBL168119 | N-[6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co. Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [125 I]ET-1 binding to Endothelin A receptor in porcine aortic membrane. | Bioorg Med Chem Lett 12: 81-4 (2001) BindingDB Entry DOI: 10.7270/Q2VX0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50105055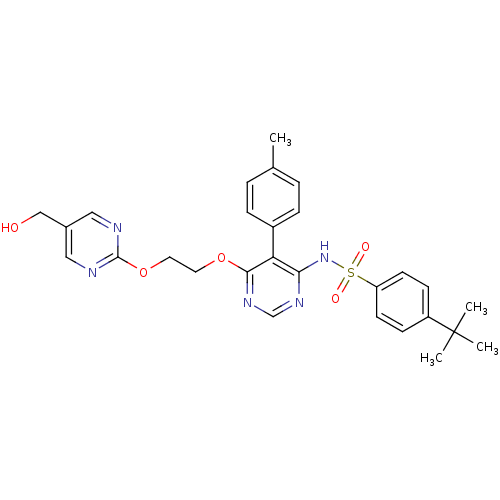 (4-tert-Butyl-N-{6-[2-(5-hydroxymethyl-pyrimidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.00260 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to endothelin A receptor in porcine aortic membrane | J Med Chem 44: 3369-77 (2001) BindingDB Entry DOI: 10.7270/Q27M08P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50105048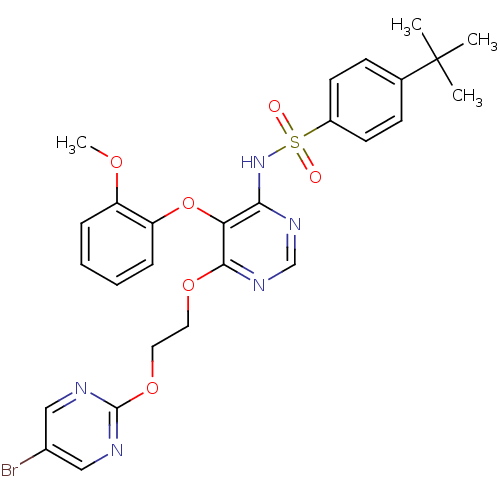 (CHEMBL112144 | N-[6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.00390 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [125I]-ET-1 binding to Endothelin A receptor in porcine aortic membrane from endothelial cells | J Med Chem 44: 3355-68 (2001) BindingDB Entry DOI: 10.7270/Q2QN662B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50105048 (CHEMBL112144 | N-[6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.00390 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co. Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [125 I]ET-1 binding to Endothelin A receptor in porcine aortic membrane. | Bioorg Med Chem Lett 12: 81-4 (2001) BindingDB Entry DOI: 10.7270/Q2VX0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50107561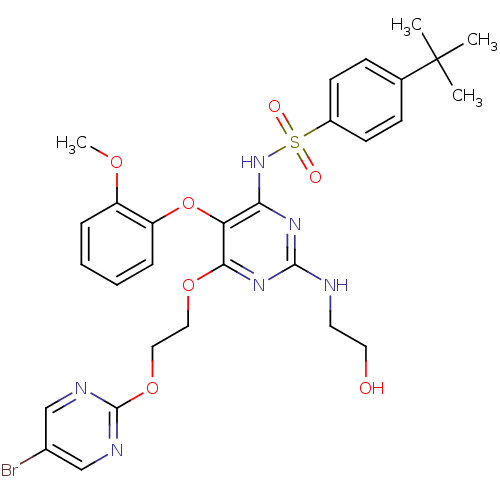 (CHEMBL168441 | N-[6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.00530 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co. Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [125 I]ET-1 binding to Endothelin A receptor in porcine aortic membrane. | Bioorg Med Chem Lett 12: 81-4 (2001) BindingDB Entry DOI: 10.7270/Q2VX0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50105051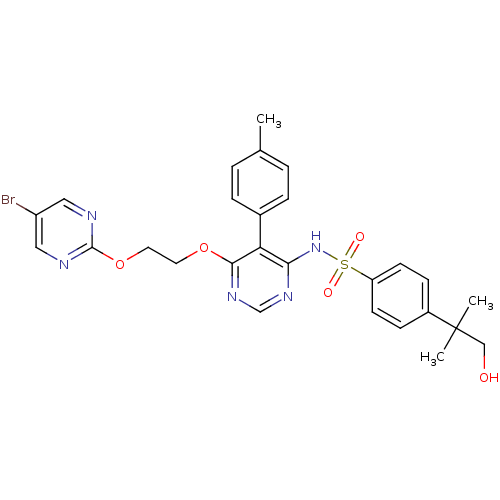 (CHEMBL112624 | N-{6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.00620 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to endothelin A receptor in porcine aortic membrane | J Med Chem 44: 3369-77 (2001) BindingDB Entry DOI: 10.7270/Q27M08P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50105047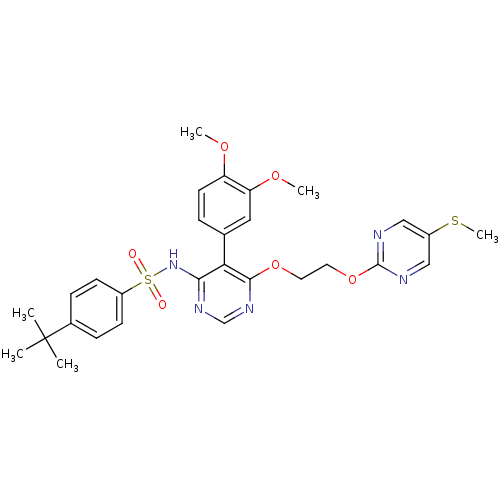 (Acetic acid ethyl ester4-tert-Butyl-N-{5-(3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00850 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [125I]-ET-1 binding to Endothelin A receptor in porcine aortic membrane from endothelial cells | J Med Chem 44: 3355-68 (2001) BindingDB Entry DOI: 10.7270/Q2QN662B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141458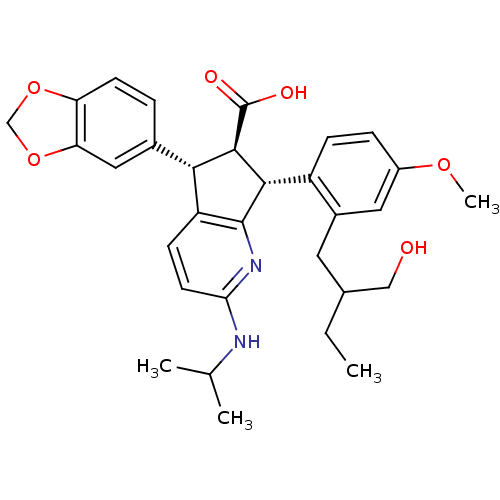 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin B receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141472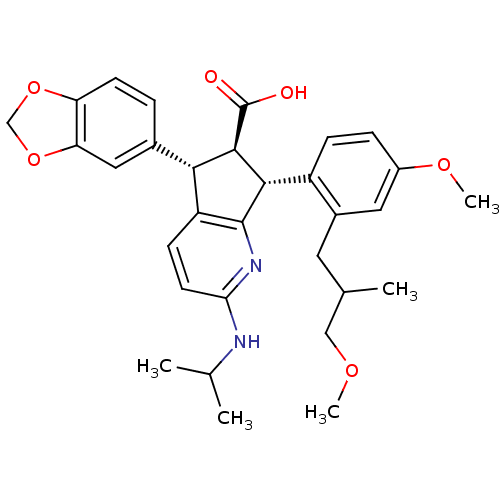 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-2-isopropylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141472 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-2-isopropylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141458 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50104986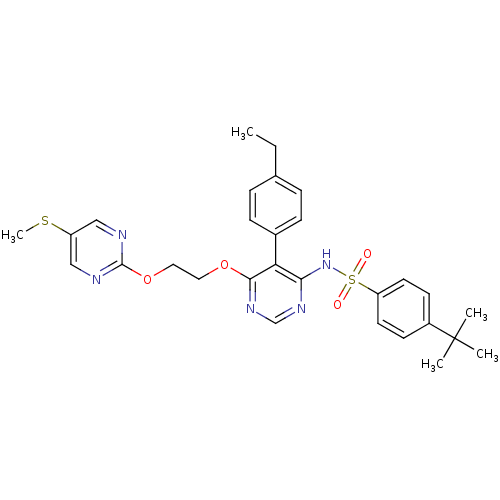 (4-tert-Butyl-N-{5-(4-ethyl-phenyl)-6-[2-(5-methyls...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [125I]-ET-1 binding to Endothelin A receptor in porcine aortic membrane from endothelial cells | J Med Chem 44: 3355-68 (2001) BindingDB Entry DOI: 10.7270/Q2QN662B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141475 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50105040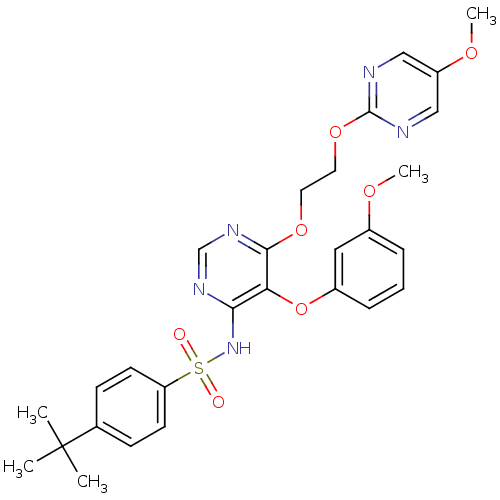 (4-tert-Butyl-N-{5-(3-methoxy-phenoxy)-6-[2-(5-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [125I]-ET-1 binding to Endothelin A receptor in porcine aortic membrane from endothelial cells | J Med Chem 44: 3355-68 (2001) BindingDB Entry DOI: 10.7270/Q2QN662B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50105026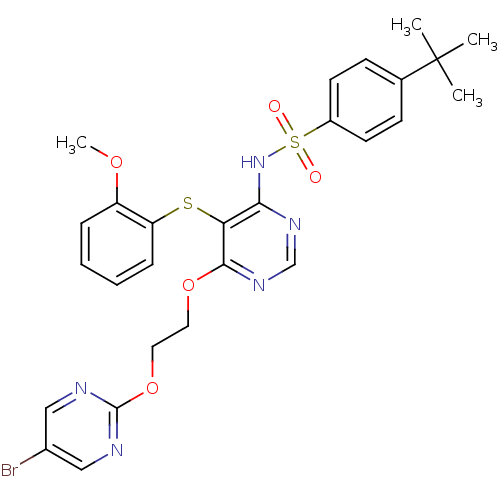 (CHEMBL115724 | N-[6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [125I]-ET-1 binding to Endothelin A receptor in porcine aortic membrane from endothelial cells | J Med Chem 44: 3355-68 (2001) BindingDB Entry DOI: 10.7270/Q2QN662B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141465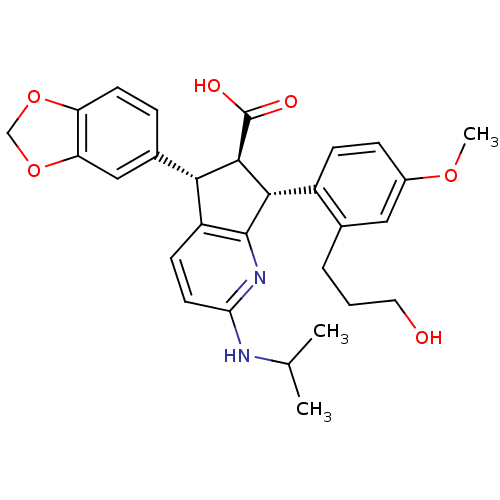 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(3-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50155246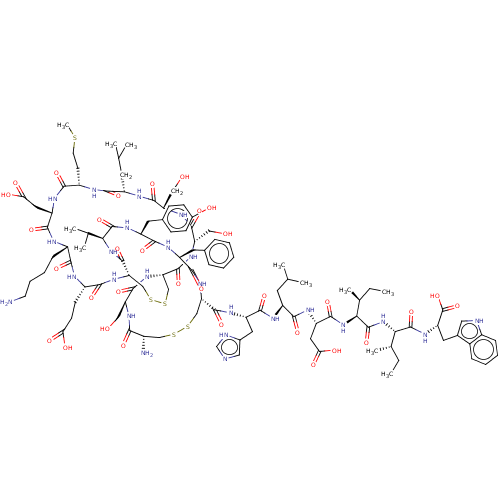 (CHEMBL3775234) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]endothelin-1 from human recombinant ETA receptor expressed in CHO cells measured after 120 mins by scintillation counting metho... | Bioorg Med Chem 25: 471-482 (2017) Article DOI: 10.1016/j.bmc.2016.11.014 BindingDB Entry DOI: 10.7270/Q2CF9S3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM392076 (US10301272, Example 7/9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Displacement of [125I]-endothelin-1 from human recombinant ETA receptor after 120 mins by scintillation counting analysis | Bioorg Med Chem Lett 28: 1446-1455 (2018) Article DOI: 10.1016/j.bmcl.2018.03.093 BindingDB Entry DOI: 10.7270/Q27W6FPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50155246 (CHEMBL3775234) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]Endothelin-1 from human recombinant Endothelin-1 receptor expressed in CHO cells | Bioorg Med Chem 24: 1793-810 (2016) Article DOI: 10.1016/j.bmc.2016.03.006 BindingDB Entry DOI: 10.7270/Q2J67JS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141468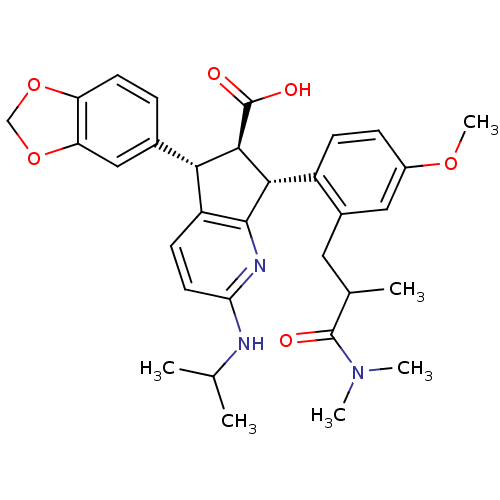 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2-dimethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141468 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2-dimethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50107567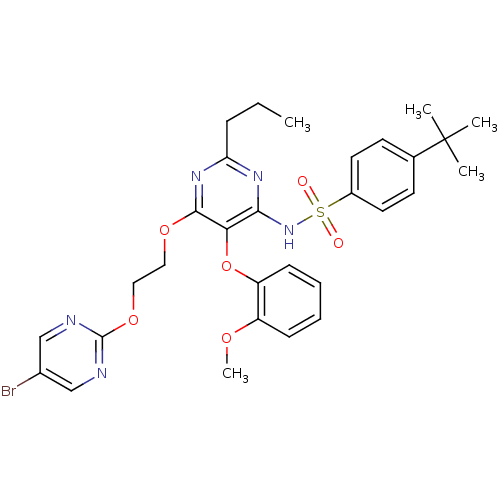 (CHEMBL170908 | N-[6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co. Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [125 I]ET-1 binding to Endothelin A receptor in porcine aortic membrane. | Bioorg Med Chem Lett 12: 81-4 (2001) BindingDB Entry DOI: 10.7270/Q2VX0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141471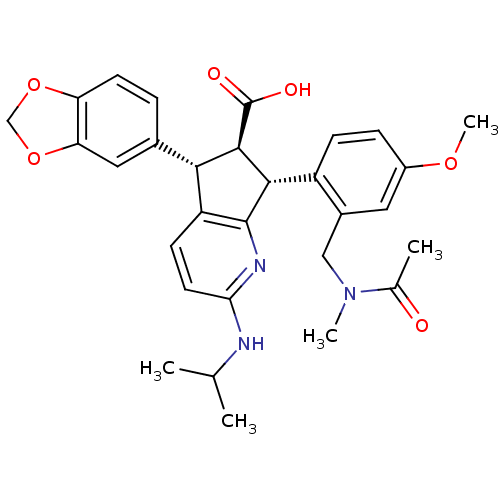 ((5S,6R,7R)-7-{2-[(Acetyl-methyl-amino)-methyl]-4-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50105025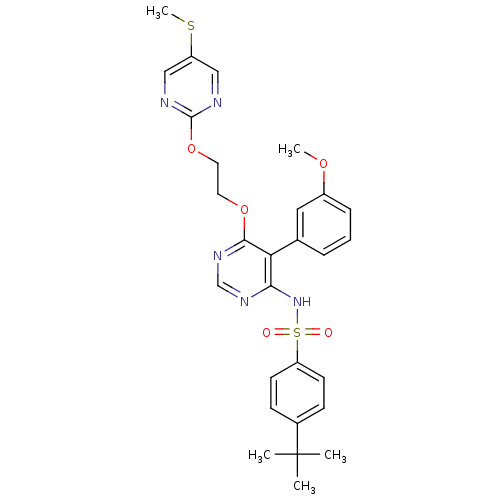 (4-tert-Butyl-N-{5-(3-methoxy-phenyl)-6-[2-(5-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [125I]-ET-1 binding to Endothelin A receptor in porcine aortic membrane from endothelial cells | J Med Chem 44: 3355-68 (2001) BindingDB Entry DOI: 10.7270/Q2QN662B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141463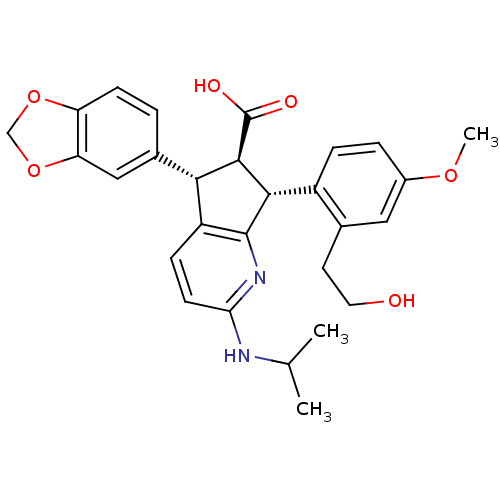 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50051007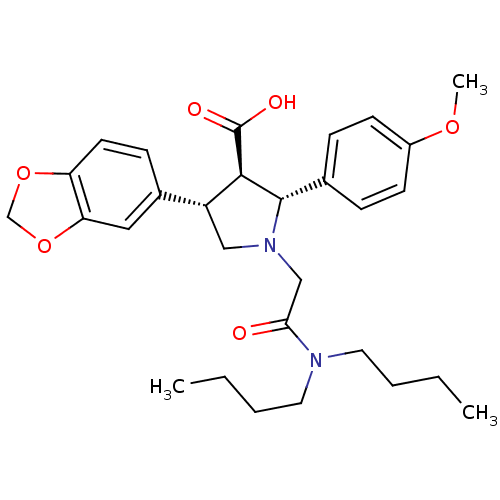 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Ability of the compound to displace endothelin ([125I]-ET-1) from human Endothelin A receptor | J Med Chem 40: 3217-27 (1997) Article DOI: 10.1021/jm970101g BindingDB Entry DOI: 10.7270/Q2C24VJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50051007 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding ability determined by the displacement of [125I]-ET-1 from the human endothelin A receptor | J Med Chem 42: 3679-89 (1999) Article DOI: 10.1021/jm990171i BindingDB Entry DOI: 10.7270/Q2125RVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50104988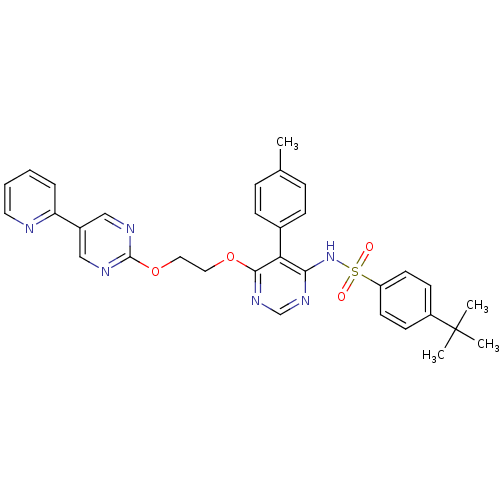 (4-tert-Butyl-N-{6-[2-(5-pyridin-2-yl-pyrimidin-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [125I]-ET-1 binding to Endothelin A receptor in porcine aortic membrane from endothelial cells | J Med Chem 44: 3355-68 (2001) BindingDB Entry DOI: 10.7270/Q2QN662B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50105054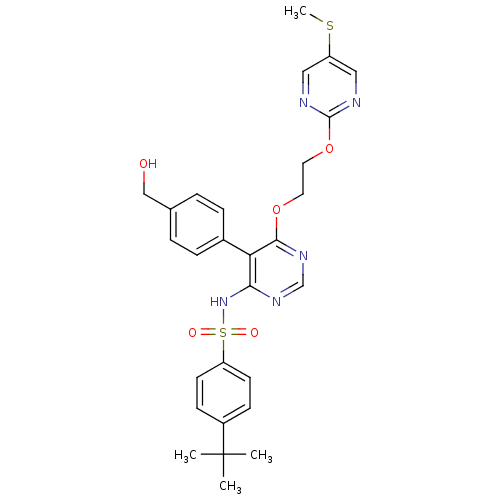 (4-tert-Butyl-N-{5-(4-hydroxymethyl-phenyl)-6-[2-(5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to endothelin A receptor in porcine aortic membrane | J Med Chem 44: 3369-77 (2001) BindingDB Entry DOI: 10.7270/Q27M08P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50105057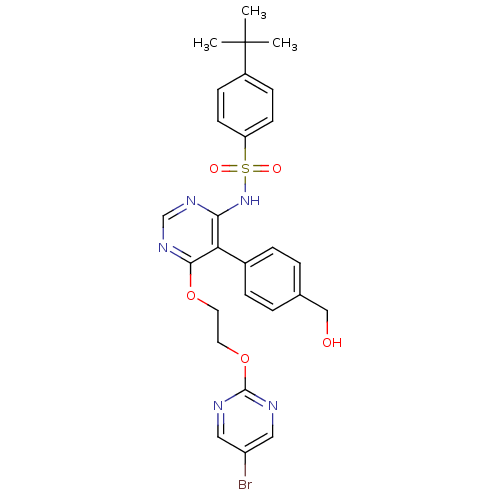 (CHEMBL324184 | N-[6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to endothelin A receptor in porcine aortic membrane | J Med Chem 44: 3369-77 (2001) BindingDB Entry DOI: 10.7270/Q27M08P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50098777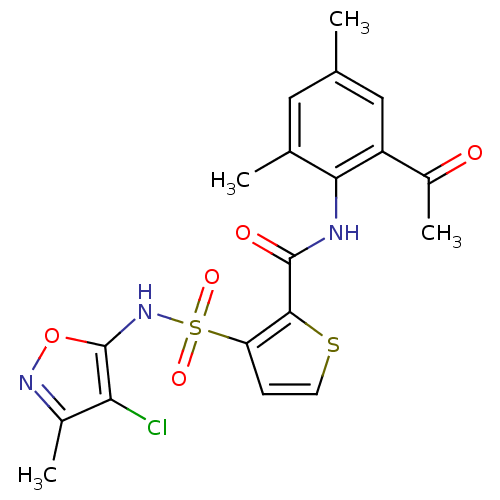 (3-(4-Chloro-3-methyl-isoxazol-5-ylsulfamoyl)-thiop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Texas Biotechnology Corporation Curated by ChEMBL | Assay Description Inhibitory concentration was determined against selective Endothelin A receptor | J Med Chem 44: 1211-6 (2001) BindingDB Entry DOI: 10.7270/Q2Z89BN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50107556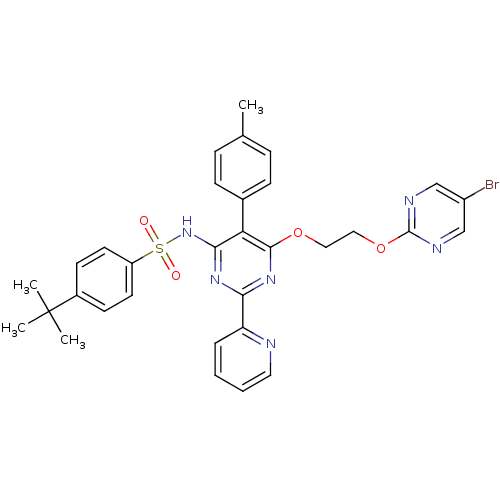 (CHEMBL170493 | N-{6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co. Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [125 I]ET-1 binding to Endothelin A receptor in porcine aortic membrane. | Bioorg Med Chem Lett 12: 81-4 (2001) BindingDB Entry DOI: 10.7270/Q2VX0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50061096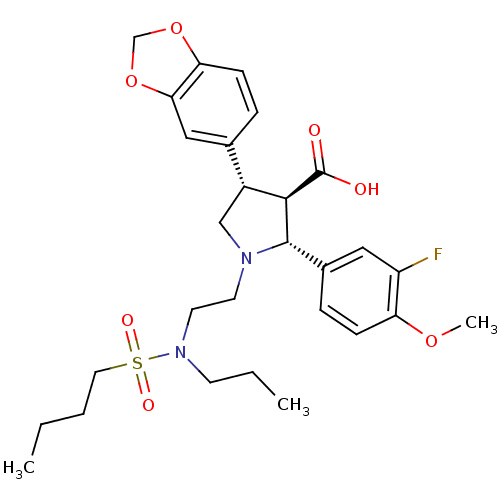 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-{2-[(butane-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding assay performed using human Endothelin A receptor (hETA) expressed in chinese hamster ovary cells(CHO). | J Med Chem 40: 3217-27 (1997) Article DOI: 10.1021/jm970101g BindingDB Entry DOI: 10.7270/Q2C24VJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50000558 (CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Inhibition of ETA receptor in human SK-N-MC cells after 60 mins by scintillation counting | J Nat Prod 72: 2172-6 (2009) Article DOI: 10.1021/np900287e BindingDB Entry DOI: 10.7270/Q2MC910T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141470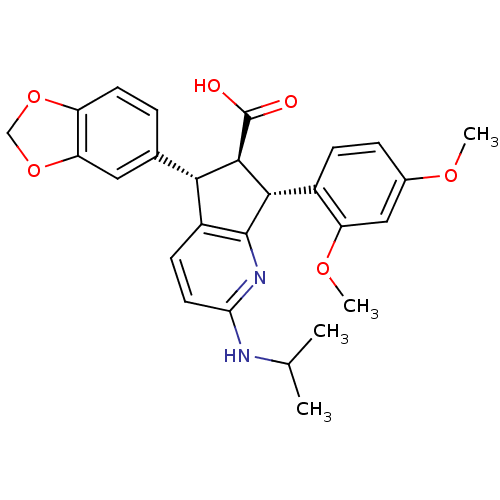 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-(2,4-dimethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141461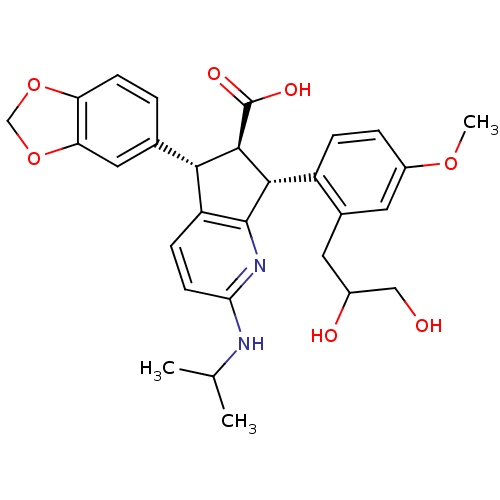 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2,3-dihyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141461 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2,3-dihyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077930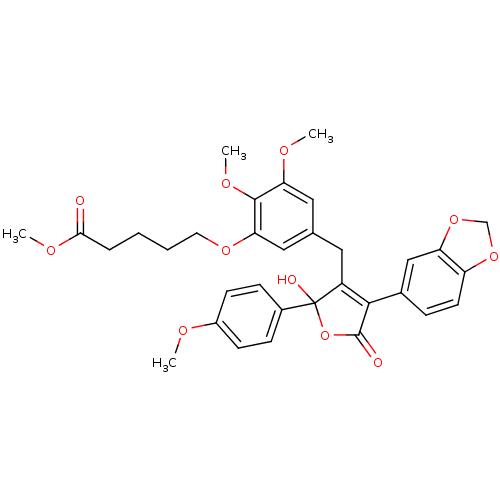 (5-{5-[4-Benzo[1,3]dioxol-5-yl-2-hydroxy-2-(4-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50104992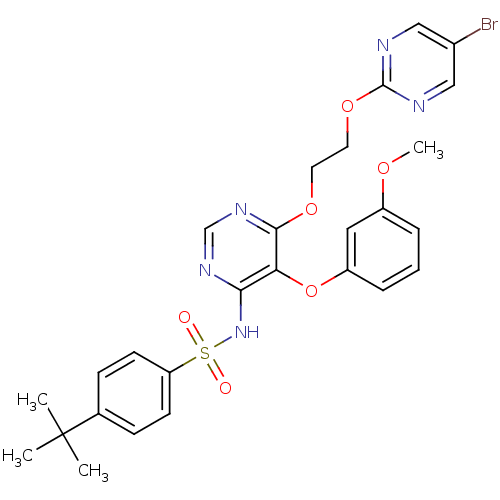 (CHEMBL114700 | N-[6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [125I]-ET-1 binding to Endothelin A receptor in porcine aortic membrane from endothelial cells | J Med Chem 44: 3355-68 (2001) BindingDB Entry DOI: 10.7270/Q2QN662B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50104992 (CHEMBL114700 | N-[6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co. Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of [125 I]ET-1 binding to Endothelin A receptor in porcine aortic membrane | Bioorg Med Chem Lett 12: 81-4 (2001) BindingDB Entry DOI: 10.7270/Q2VX0FSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50061078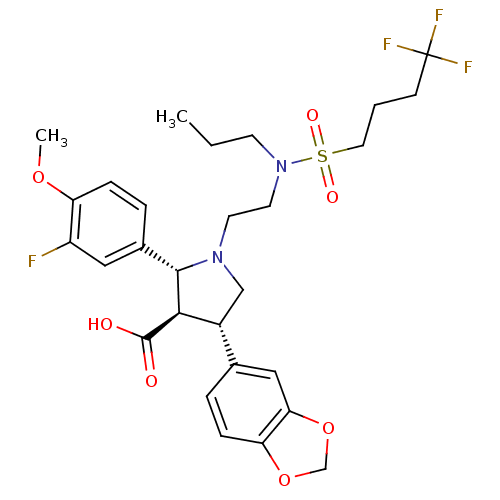 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-2-(3-fluoro-4-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding assay performed using human Endothelin A receptor (hETA) expressed in chinese hamster ovary cells(CHO). | J Med Chem 40: 3217-27 (1997) Article DOI: 10.1021/jm970101g BindingDB Entry DOI: 10.7270/Q2C24VJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50051007 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) Curated by ChEMBL | Assay Description Displacement of [125I]ET-1 from human ET-A receptor expressed in CHO cell membrane | J Med Chem 59: 8168-88 (2016) Article DOI: 10.1021/acs.jmedchem.5b01781 BindingDB Entry DOI: 10.7270/Q22N55RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141467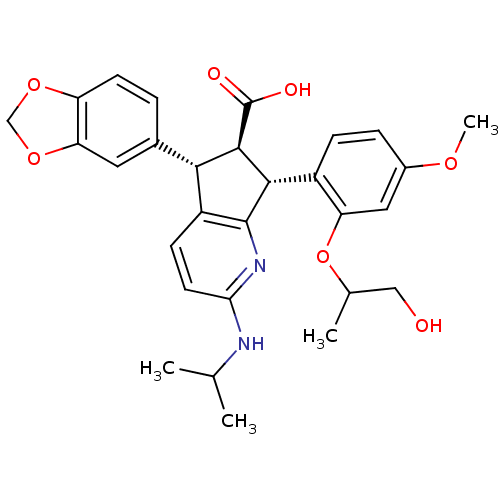 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141467 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141460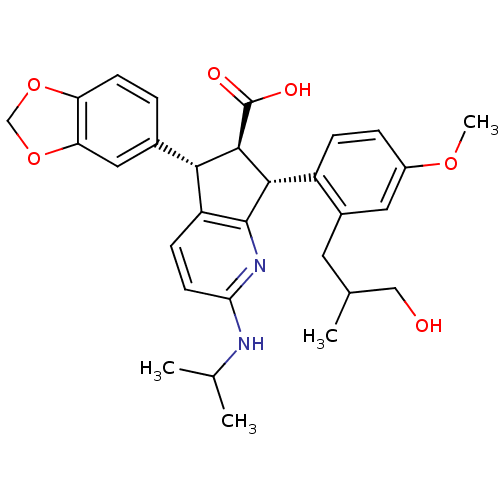 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(3-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141460 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(3-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin B receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1920 total ) | Next | Last >> |